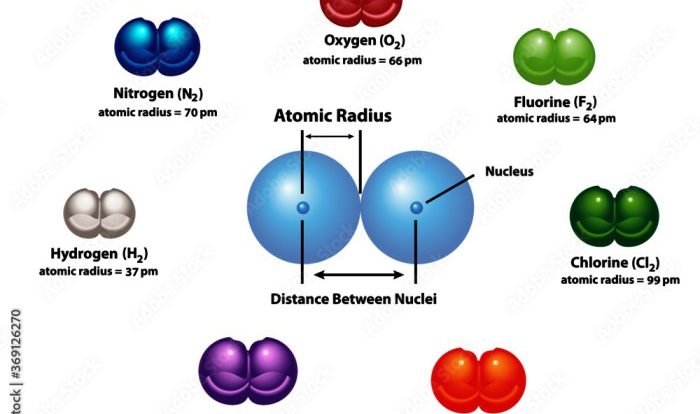
Delve into the fascinating world of chemistry with our meticulously crafted grams to moles and moles to grams worksheet. This comprehensive guide empowers you to navigate the intricate relationship between mass and amount of substance with ease.
Embark on a journey of discovery as we unravel the concepts of moles, molar mass, and the conversion formulas that seamlessly connect these two fundamental units of measurement.
Introduction to Moles and Grams: Grams To Moles And Moles To Grams Worksheet

In chemistry, it is crucial to quantify the amount of substances involved in chemical reactions and other processes. The mole and the gram are two essential units of measurement that play a vital role in this quantification.
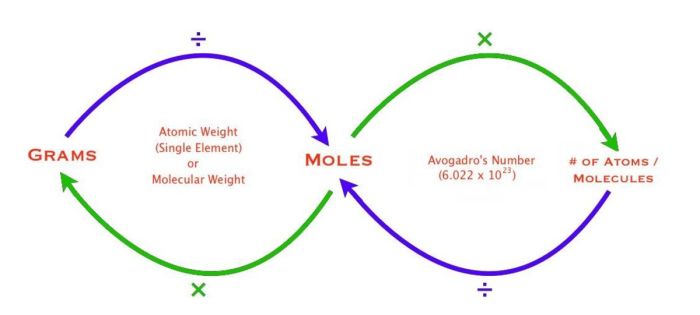
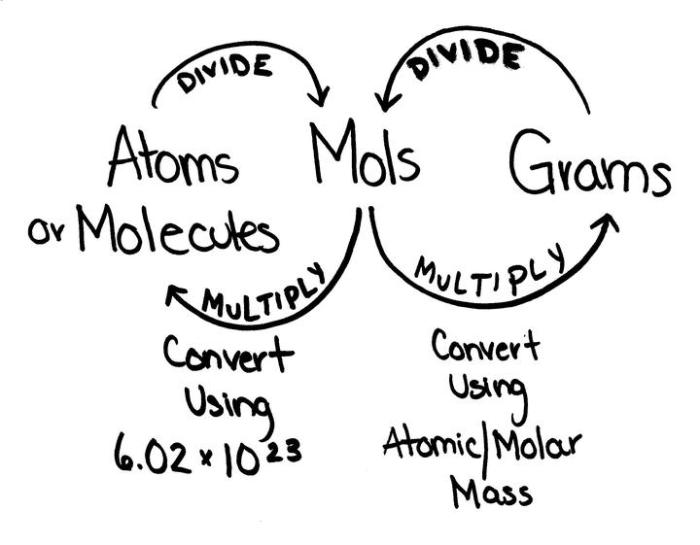
The mole, denoted by the symbol “mol,” is the SI unit for the amount of substance. It represents a specific number of entities, known as Avogadro’s number, which is approximately 6.022 × 10 23.
On the other hand, the gram, denoted by the symbol “g,” is the SI unit for mass. It represents the mass of a substance, which is the measure of its inertia and the amount of matter it contains.
Converting Grams to Moles
To convert grams to moles, we use the following formula:
moles = grams / molar mass
The molar mass of a substance is its mass per mole, expressed in grams per mole (g/mol). It is a characteristic property of each substance and can be found using the periodic table or reference tables.
For example, the molar mass of water (H 2O) is 18.015 g/mol. This means that 18.015 grams of water is equal to 1 mole of water.
| Substance | Molar Mass (g/mol) | Grams | Moles |
|---|---|---|---|
| Water (H2O) | 18.015 | 36.03 | 2 |
| Sodium chloride (NaCl) | 58.44 | 116.88 | 2 |
| Glucose (C6H12O6) | 180.16 | 360.32 | 2 |
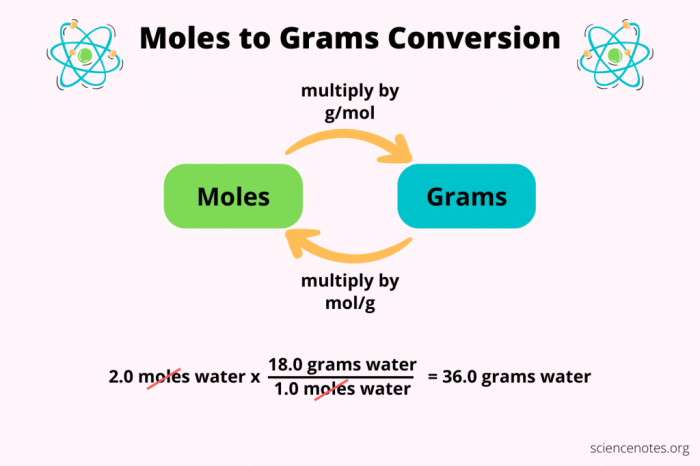
Converting Moles to Grams, Grams to moles and moles to grams worksheet
To convert moles to grams, we use the following formula:
grams = moles
molar mass
The molar mass, as mentioned earlier, is the mass per mole of a substance. It is essential in this conversion as it provides the necessary information to determine the mass corresponding to a given number of moles.
For example, if we have 2 moles of water (H 2O), we can calculate the mass as follows:
grams = 2 moles
18.015 g/mol = 36.03 g
| Substance | Molar Mass (g/mol) | Moles | Grams |
|---|---|---|---|
| Water (H2O) | 18.015 | 2 | 36.03 |
| Sodium chloride (NaCl) | 58.44 | 2 | 116.88 |
| Glucose (C6H12O6) | 180.16 | 2 | 360.32 |
Frequently Asked Questions
What is the significance of molar mass in converting grams to moles?
Molar mass acts as the bridge between the mass and the amount of substance. It allows us to determine the number of moles present in a given mass of a substance and vice versa.
Can you provide an example of converting 50 grams of sodium chloride to moles?
To convert 50 grams of sodium chloride to moles, we use the formula: moles = grams / molar mass. The molar mass of sodium chloride is 58.44 g/mol. Therefore, moles = 50 g / 58.44 g/mol = 0.855 moles.