Draw all reasonable resonance structures for the following species. Resonance structures are a way of representing the electronic structure of a molecule or ion by showing all possible arrangements of the electrons within the molecule or ion. They are used to show the different ways that the electrons can be distributed around the atoms in a molecule or ion, and to predict the stability and reactivity of the molecule or ion.
Resonance structures are important because they can help us to understand the electronic structure of molecules and ions, and to predict their properties. They can also be used to explain the reactivity of molecules and ions, and to design new molecules and ions with specific properties.
1. Introduction
In chemistry, resonance structures are alternative Lewis structures that represent the same molecule or ion. They are used to describe the electronic structure of molecules that have multiple bonds and/or lone pairs of electrons.
Resonance structures are not real structures, but rather a way of representing the delocalization of electrons within a molecule. The actual structure of a molecule is a hybrid of all of its resonance structures.
Drawing resonance structures can help us to understand the electronic structure of molecules and to predict their stability and reactivity.
2. Methods for Drawing Resonance Structures
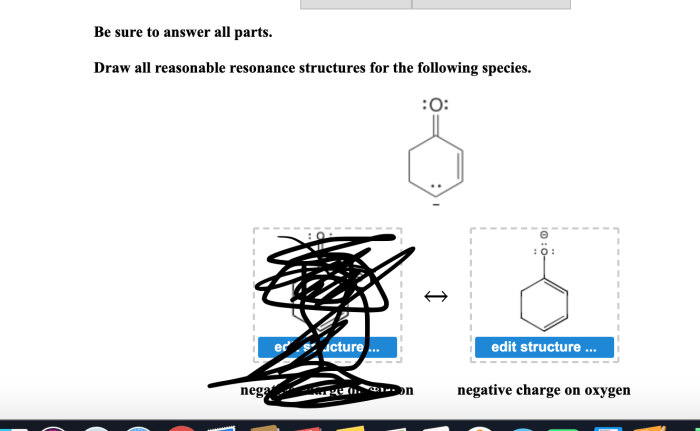
To draw resonance structures, we need to follow these steps:
- Identify all of the atoms in the molecule.
- Draw a Lewis structure for the molecule.
- Identify all of the atoms that have multiple bonds or lone pairs of electrons.
- Move electrons around to create new resonance structures.
- Check that all of the resonance structures have the same number of electrons and the same overall charge.
Here are some tips for identifying and selecting the most important resonance structures:
- The most important resonance structures are those that have the lowest energy.
- Resonance structures that have more covalent bonds are more stable than those with fewer covalent bonds.
- Resonance structures that have more negative charges on electronegative atoms are more stable than those with fewer negative charges on electronegative atoms.
3. Examples of Resonance Structures
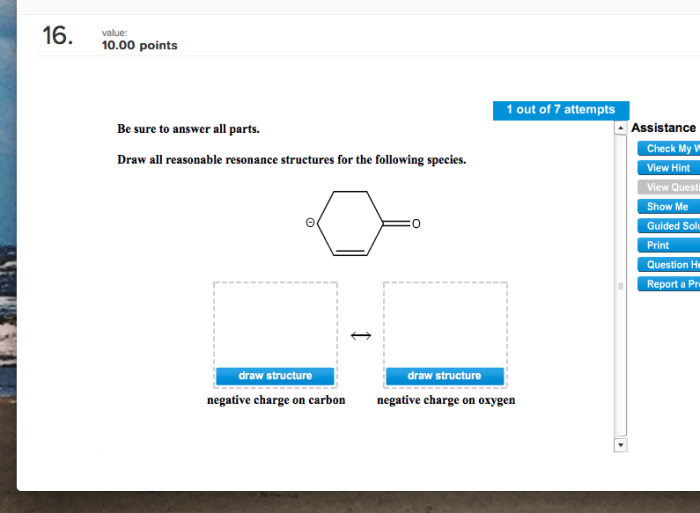
Here are some examples of resonance structures:
Carbon dioxide (CO2)
Carbon dioxide has two resonance structures:

Benzene (C6H6)
Benzene has six resonance structures:

Nitrate ion (NO3-)
The nitrate ion has three resonance structures:

4. Applications of Resonance Structures
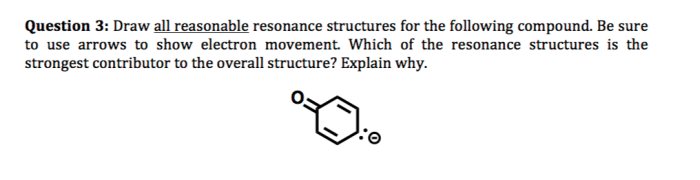
Resonance structures can be used to:
- Predict the stability of molecules
- Determine the reactivity of molecules
- Understand the electronic structure of molecules
For example, resonance structures can be used to explain why benzene is so stable. Benzene has six resonance structures, which means that the electrons in the benzene ring are delocalized. This delocalization of electrons makes benzene more stable than other molecules with the same number of carbon atoms.
5. Advanced Topics in Resonance Structures
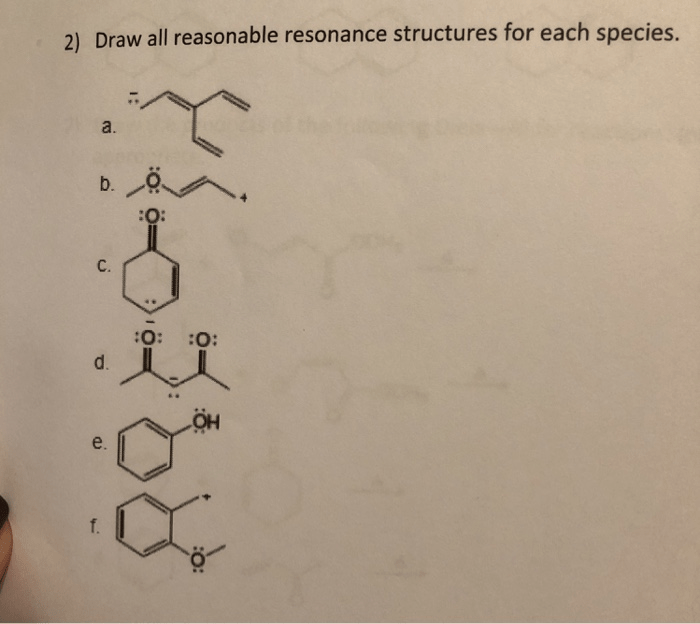
In addition to the basic concepts of resonance, there are also some more advanced topics that can be explored.
Resonance hybridization, Draw all reasonable resonance structures for the following species.
Resonance hybridization is a concept that is used to explain the bonding in molecules that have multiple resonance structures. Resonance hybridization involves the mixing of atomic orbitals to form new hybrid orbitals that are used to bond the atoms in the molecule.
Resonance structures and transition metal complexes
Resonance structures can also be used to explain the properties of transition metal complexes. Transition metal complexes are molecules that contain a metal ion that is bonded to a ligand. The ligands can be either neutral or charged, and they can donate or accept electrons to the metal ion.
Resonance structures can be used to explain the bonding in transition metal complexes and to predict their stability and reactivity.
Helpful Answers: Draw All Reasonable Resonance Structures For The Following Species.
What is a resonance structure?
A resonance structure is a way of representing the electronic structure of a molecule or ion by showing all possible arrangements of the electrons within the molecule or ion.
Why are resonance structures important?
Resonance structures are important because they can help us to understand the electronic structure of molecules and ions, and to predict their properties. They can also be used to explain the reactivity of molecules and ions, and to design new molecules and ions with specific properties.
How do I draw resonance structures?
To draw resonance structures, you need to follow these steps:
- Draw the Lewis structure of the molecule or ion.
- Identify all of the atoms that have lone pairs of electrons.
- Move one of the lone pairs of electrons to a соседний atom that has an empty orbital.
- Repeat steps 2 and 3 until you have drawn all of the possible resonance structures.